HAVE YOU EVER WONDER WHAT THIS PICTURE OR SIMILAR PICTURES LIKE THIS ONE MEAN? LETS LEARN SOME CHEMISTRY!!!!!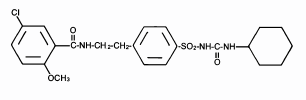
Glyburide : A anti-diabetic drug. This diagram just gives the locations of all of the atoms in this molecule. (double lines = Carbon), (Hydrogen only comes out when its bonded with another atom other than Carbon).
This diagram is called the "Structural Formula" or the "Lewis Structure"
TERMS:
Structural Formula - A formula in which the connections of the atoms and groups of atoms, as well as their kind of number, are indicated.
Lewis Structure - Named after Gilbert N. Lewis who introduced it in his 1916 article The Atom and the Molecule. The Lewis Structure are diagrams that show the bonding between atoms of a molecule, and the lone pairs of electrons that may exist in the molecule. A Lewis Structure can be drawn for any covalently-bonded molecule, as well as the coordination compounds.
Atom - The atom is the basic unit of matter consisting of a dense, central nucleus surrounded by a cloud of negatively charged electrons.
Electrons - The electron is a subatomic particle that carries a negative electric charge.
Ion - An ion is a atom or molecule in which the total number of electrons is not equal to the total number of protons giving it a net positive or negative electrical charge.
Molecules - An electrically neutral group of at least two atoms in a definite arrangement held together by very strong chemical bonds.
Covalent Bond - Is a form of chemical bonding that is characterized by the sharing of pairs of electrons between atoms and other covalent bonds. In short, the attraction to repulsion stability that forms between atoms when they share electrons is known as covalent bonding.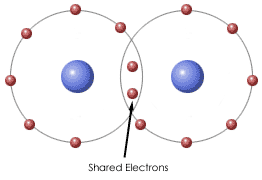
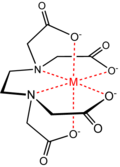
Coordination Complex / Coordination Compounds - Is a structure consisting of a central atom or ion, bonded to a surrounding array of molecules or anions (ligands, complexing agents). The atom within a ligand that is directly bonded to the central atom or ion is called the donor atom. A ligand donates at least one pair of electrons to the central atom/ion. Compounds that contain a coordination complex is called coordination compounds. The central atom or ion, together with all ligands form the coordination sphere.